Jan. 27, 2020:
A new Big Ten Cancer Research Consortium phase II study will test the combination of gedatolisib and talazoparib in advanced triple-negative or BRCA 1/2-mutated HER2-negative breast cancer in men and women.
Breast cancer is the most common cancer in women, with more than 266,000 new cases in 2018. Breast cancer in men is less common, with more than 2,400 new cases annually. About 15 percent of all invasive breast cancers diagnosed in the United States each year are triple-negative. Triple-negative breast cancers don’t express the estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER-2). Inherited gene mutations in BRCA1 or 2 are responsible for about 5 to 10 percent of breast cancers in the United States.
People with advanced triple-negative breast cancer have fewer treatment options and the primary treatment for triple-negative breast cancer is chemotherapy. Exploring non-chemotherapy options for treatment may uncover new ways to treat this disease.
The goal of the study, BTCRC-BRE18-337, “Phase 2 Trial with Safety Run-In of Gedatolisib Plus Talazoparib in Advanced Triple Negative or BRCA1/2 Positive, HER2 Negative Breast Cancers,” is to identify the safe doses for combining gedatolisib and talazoparib, and to assess the efficacy of the combination.
The study is open to accrual at the University of Wisconsin Carbone Cancer Center in Madison, Wis.; the University of Illinois Cancer Center in Chicago; and the University of Iowa Holden Comprehensive Cancer Center in Iowa City.
“What we’re really trying to figure out is how many people’s tumors respond, how long those responses last, and if that is more than we anticipated based on previous studies,” said Kari B. Wisinski, MD (pictured left), sponsor-investigator of the study and a medical oncologist at the University of Wisconsin Carbone Cancer Center.
Talazoparib is a targeted therapy that blocks an enzyme called poly (ADP-ribose) polymerase, or PARP, which is involved in cell DNA repair. In cancer, blocking PARP may prevent cancer cells from repairing damaged DNA, causing the cells to die.
Gedatolisib is an investigational compound, thought to block two receptors, PI3K and mTOR. Activation of the PI3K/mTOR pathway is common in breast cancers and helps cells grow, survive, and become resistant to chemotherapy and radiotherapy. By blocking the PI3K and mTOR kinases, gedatolisib may inhibit the growth of certain cancer cells, leading to cell death.
Some researchers hypothesize talazoparib and gedatolisib may have more benefits as a combined therapy than taking either drug alone.
“Gedatolisib is a dual inhibitor,” Dr. Wisinski emphasized. “That’s important because we think dual inhibitors might have a more potent effect rather than a drug that inhibits one or the other. The idea here is that PI3-Kinase pathway inhibition might increase homologous recombination deficiency in cancer cells, hopefully sensitizing the tumors to PARP inhibitors.”
Talazoparib is approved by the U.S. Food and Drug Administration (FDA) to treat adult patients with deleterious or suspected deleterious gBRCA-mutated HER2-negative, locally advanced or metastatic breast cancer. Patients must be selected for therapy based on an FDA-approved companion diagnostic for talazoparib. Gedatolisib is not FDA-approved to treat any disease. The combination of talazoparib and gedatolisib is not FDA-approved and should be considered investigational.
Testing of this combination therapy will be conducted in two parts. The first part will identify the safe dose of talazoparib and gedatolisib, where up to 18 people can participate. The second part will determine if the combination therapy has an effect on triple negative breast cancer, where up to 36 people can participate. In total, 54 people may participate in this study.
The combination therapy of talazoparib and gedatolisib will be administered in four-week cycles. Talazoparib is a pill that participants will take daily. Gedatolisib will be given every week intravenously. Participants may take this combined therapy until their cancer worsens or side effects become intolerable.
Additionally, blood will be collected from participants to study tumor DNA.
“We’re going to look at some characteristics in the tumor called HRD — Homologous Recombination Deficiency — with a test to try to see if we can identify which patients seem to benefit, or which tumors have predisposition to benefiting from this treatment,” said Dr. Wisinski.
Sneha Phadke, DO (pictured right), co-investigator of the study and a medical oncologist at University of Iowa, has been involved in the study since its beginning.
“Unfortunately, patients with triple negative breast cancer still have limited therapy options,” Dr. Phadke said. “I am excited to be involved in this study which explores the possibility of non-chemotherapy treatments. With the help of the Big Ten Cancer Research Consortium, we hope to more efficiently recruit patients from multiple cancer centers, which should allow us to obtain results and help patients more quickly.”
“One of the things I enjoy about the Big Ten Cancer Research Consortium is that it encourages involvement of the co-investigators from other institutions,” Dr. Wisinski said. “Dr. Phadke is a natural fit for this study.”
To qualify for this study, patients must be adults age 18 or older with advanced triple-negative or BRCA 1/2-mutated HER2-negative breast cancer.
For more information about this research study, including full eligibility requirements, visit clinicaltrials.gov (study #NCT03911973).
Support for this study is provided by Pfizer Inc.
About the Big Ten Cancer Research Consortium: The Big Ten Cancer Research Consortium was created in 2013 to transform the conduct of cancer research through collaborative, hypothesis-driven, highly translational oncology trials that leverage the scientific and clinical expertise of Big Ten universities. The goal of the Big Ten Cancer Research Consortium is to create a unique team-research culture to drive science rapidly from ideas to new approaches to cancer treatment. Within this innovative environment, today’s research leaders collaborate with and mentor the research leaders of tomorrow with the unified goal of improving the lives of all patients with cancer.
About the Big Ten Conference: The Big Ten Conference is an association of world-class universities whose member institutions share a common mission of research, graduate, professional and undergraduate teaching and public service. Founded in 1896, the Big Ten has sustained a comprehensive set of shared practices and policies that enforce the priority of academics in the lives of students competing in intercollegiate athletics and emphasize the values of integrity, fairness and competitiveness. The broad-based programs of the 14 Big Ten institutions will provide over $200 million in direct financial support to more than 9,800 students for more than 11,000 participation opportunities on 350 teams in 42 different sports. The Big Ten sponsors 28 official conference sports, 14 for men and 14 for women, including the addition of men’s ice hockey and men’s and women’s lacrosse since 2013. For more information, visit www.bigten.org.
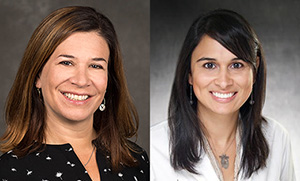












Subscribe to the Big Ten CRC Newsletter X
X Facebook
Facebook YouTube
YouTube